The hepatotoxic potential of new drug candidates remains difficult to predict based on animal safety studies. This is mostly due to species-specific differences in drug metabolism pathways. Isolated human hepatocytes remain the most commonly used alternative. Problems, however, are encountered because of limited availability of liver donors, as well as progressive dedifferentiation and short lifespan of primary hepatocytes in an in vitro setting.
Consequently, stem cells have become an attractive cell source to develop human-relevant in vitro models for safety assessment of new chemical entities, and for tissue engineering. Distinct stem cell populations, either derived from embryonic or adult tissues, are currently available. Due to ethical controversy and legislative restrictions associated with embryonic stem cells (ESCs), our team focuses on natural stem cell pools residing in postnatal human and animal tissues. Although they all share a multipotent character, postnatal stem cells isolated from distinct tissue niches manifest large differences in inherent plasticity and differentiation potential towards specific cell types such as hepatocytes.
Therefore, we are currently exploring the hepatogenic differentiation potential of:
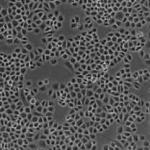
Discover how we develop an in vitro liver model for drug safety studies by in vitro expansion and hepatogenic differentiation of rat bile duct-derived stem cells.
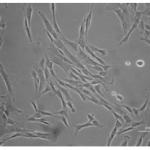
Discover how we develop an in vitro liver model for drug safety studies by in vitro expansion and hepatogenic differentiation of Wharton's jelly-derived stem cells
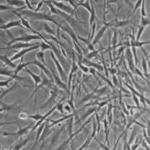
Discover how we develop an in vitro liver model for drug safety studies by in vitro expansion and hepatogenic differentiation of foreskin-derived stem cells.
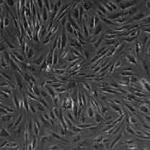
Discover how adult human subcutaneous adipose tissue harbors a multipotent stem cell population, the so-called human adipose tissue-derived mesenchymal stromal cells (AT-MSCs).
