In vivo and in vitro animal models are commonly used to identify the hepatotoxic potential of new drug candidates, but fail to do so due to interspecies differences in drug metabolism pathways. On the other hand, human primary hepatocytes are scarce and, similarly to primary animal cells, suffer from gradual dedifferentiation in in vitro setting.
Our group offers an alternative to primary human and animal hepatocytes:
Human-relevant hepatic system derived from adult stem cells
A protocol was established based on the state of the art knowledge of liver development and epigenetic mechanisms of gene regulation. Accordingly, to obtain human hepatocyte-like cells, postnatal stem cells are exposed to specific growth factors and cytokines in a sequential and strictly time-bound manner. By addition of epigenetic modifiers the acquisition of a hepatocyte-like gene expression pattern is facilitated.
Using this protocol, hepatocyte-like cells are obtained that adopt cuboidal cell morphology, typical for mature hepatocytes, and express among others:
- liver-enriched transcription factors,
- the key hepatic protein and major plasma proteins (Fig 1).
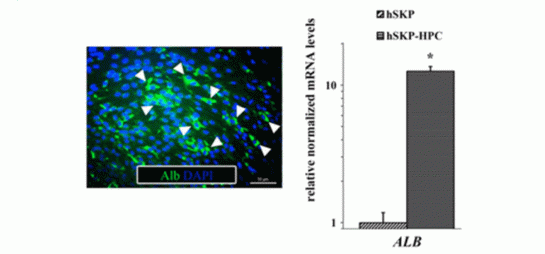
Fig. 1 Albumin
Moreover, these hepatocyte-like cells demonstrate the functionality of:
- drug transporters and drug metabolizing enzymes,
- urea and albumin secretion ability.
Yet the most importantly, hepatocyte-like cells generated by this methodology respond in a similar way to prototypical liver toxicants, e.g. acetaminophen, as primary human hepatocytes in culture.
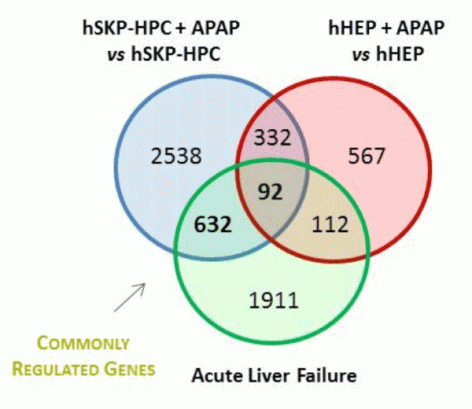
|
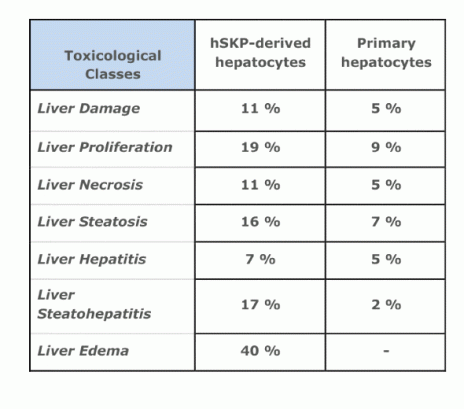
|
Advantages
- not hindered by species-specific barriers and thus applicable to human and animal postnatal stem/progenitor cell cultures,
- use of easily accessible and ethically uncontroversial stem cell sources,
- initial stem cell populations can be expanded according to the needs,
- successful hepatic differentiation of postnatal stem/progenitor cells derived from various tissues, including bone marrow, adipose tissue, skin and bile duct,
- no genetic manipulation required,
- proved applicability of obtained hepatocyte-like cells in a 'real life' toxicological assay.
IP Status
Granted EP patent “Differentiation of stem cells and stabilization of phenotypical properties of primary cells, EP1824965 (B1), US patent pending, US2009/0130064 (A1)
Market opportunities
Product development asks for functional and stable in vitro models for safety and/or efficacy testing. Therefore, hepatocyte-like cells derived by means of the above described hepatic differentiation technology could have high impact on:
- the cosmetic industry, where animal testing and marketing bans became final in March 2013, irrespective of the availability of alternative non-animal tests,
- the pharmaceutical industry, where in vitro methods become key for early decision making in the drug development process due to their speed, high throughput and cost-effectiveness,
- the chemical industry, where safety testing under REACH is associated with substantial financial costs and high animal consumption.
What are we looking for?
- Out-licensing of the hepatogenic differentiation technology (EP1824965 (B1)),
- Setting up joint R&D projects with industrial partners to develop toxicological assays based on hepatocyte-like cells obtained with our hepatogenic differentiation technology,
- Providing expertise in the area of (hepatic) stem cell differentiation technologies on the fee-for-service or collaborative basis.
Customer-tailored solutions are also discussable.
Interested parties can contact:
