TOXIN: Non-Animal Methodologies for Toxicity Testing of Chemical Compounds
Currently, there are no validated animal-free replacement methods to assess repeated dose toxicity. This poses serious problems for the development of new chemical compounds across a variety of sectors, in particular cosmetics, where animal use is now fully banned in the EU. Advancing safety assessment of chemicals without the use of animal testing is thus urgently required to ensure a level of safety that is at least equivalent to that obtained through animal testing.
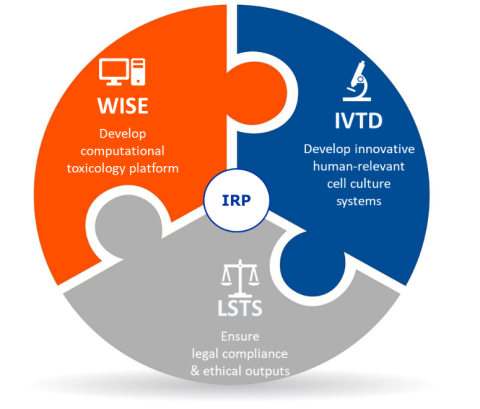
The goal of this IRP is to establish non-animal based, human-relevant strategies to assess repeated dose toxicity. This will be achieved through the unique combination of expertise in in vitro experimental toxicology (IVTD), computational information systems (WISE), and ethics and legislation (LSTS).
The objectives are:
- To develop human cell culture systems that accurately predict liver toxicity;
- To establish integrated computational approaches that optimally support the in vitro research;
- To research ethical concerns that may arise as a result of the new methods;
- To ensure that the developed non-animal based strategies are in line with all legal requirements;
- To train a new generation of interdisciplinary researchers in the different scientific domains involved in the IRP;
- To ensure visibility of the developed strategies.
This approach can be extrapolated to any other organ and/or type of chemical and will trigger follow-up initiatives across diverse industrial sectors, ensuring continuity of the IRP.
Start Date: 01-11-2019
End Date: 31-10-2024
Research Groups Involved
IVTD - In Vitro Toxicology and Dermato-Cosmetology
WISE - Web & Information Systems Engineering
LSTS - Law, Science, Technology & Society
Researchers Involved
IVTD: Tamara Vanhaecke (promotor), Mathieu Vinken (co-promotor), Joery De Kock (co-promotor)
WISE: Olga De Troyer (co-promotor)
LSTS: Paul Quinn (co-promotor)
Funded By
VUB - IRP
Related Publications
|
Year |
Publication Title |
|
2021 |
Sanctorum A., Riggio J., Sepehri S., Arnesdotter E., Vanhaecke T. and De Troyer O. (2021) A Jigsaw-based end-user tool for the development of ontology-based knowledge bases. Proceedings of IS-EUD 2021, 8th International Symposium on End-User Development, July 2021 |
|
2020 |
Gustafson E., Debryune C., De Troyer O., Rogiers V., Vinken M.* and Vanhaecke T.* (2020) Screening of repeated dose toxicity data in safety evaluation reports of cosmetic ingredients issued by the Scientific Committee on Consumer Safety between 2009 and 2019. Archives of Toxicology 94(11): 3723-3735. (*shared last authors). |
|
2020 |
Debruyne C., Riggio J., Gustafson E., O’Sullivan D., Vinken M., Vanhaecke T. and De Troyer O. (2020) 14th (2020) Facilitating data curation: a solution developed in the toxicology domain. IEEE International Conference on Semantic Computing (ICSC 2020). IEEE: 315-320. |
