LIVER DISEASE THERAPEUTICS
Advanced liver therapeutics (ALT) – Prof. Joery De Kock
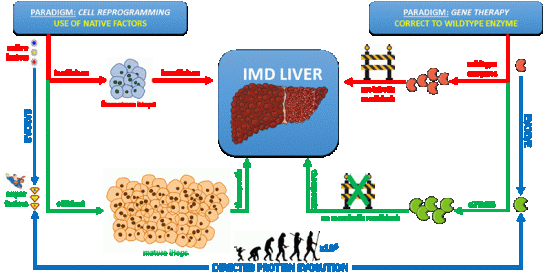
Inherited metabolic disorders (IMD) of the liver are a heterogeneous group of rare diseases caused by a single defective enzyme in a metabolic pathway of hepatocytes. This often leads to the accumulation of toxic metabolites and the highly specific destruction of liver cells. For most IMD, the ability to replace a portion of the defective hepatocytes or to correct the deficient enzyme in a subset of liver cells would suffice to produce a clinical cure. Transplantation of human hepatocytes represents a genuine therapy for IMD, but is limited by a shortage of donors and the potential need for life-long immune suppression. For some IMD, simply replacing or correcting the deficient enzyme in a subset of hepatocytes is not sufficient to provide a cure due to the cell-autonomous pathophysiology of the disease.
Major advancements in the treatment of IMD are currently hampered by two important paradigms:
Paradigms that hamper the advancement of IMD treatment.
i) The medical field holds on to the fact that deficient enzymes should be replaced or corrected to their original functional wildtype state, but fails to consider that available gene editing and cell replacement therapies are not efficient enough to replenish all copies of the dysfunctional gene with functional counterparts in order to completely normalize the patient’s metabolome.
ii) Cell reprogramming strategies are solely designed to use native reprogramming factors, but don’t consider that these factors weren’t intended by nature to direct cell-fate conversions that do not naturally occur during development. They are therefore not optimally adapted to efficiently perform this task, preventing the large scale production of so-called induced hepatocyte-like cells (iHeps).
In this research track, developed and supervised by Prof. Joery De Kock, we develop solutions to these problems by implementing a versatile technology called “directed protein evolution”. This technology
allows to artificially accelerate evolution by more than six orders of magnitude through iterative rounds of smart mutagenesis and selection, resulting in new proteins with superior properties.
Currently three PhD projects are ongoing:
- Preclinical development of a novel gene-based therapy for hereditary tyrosinemia type 1 (link 6)
- Biomarker discovery and advanced monitoring of new cell and gene-based therapies for hereditary tyrosinemia type 1 (>link 7)
- Improve the conversion of skin-derived fibroblasts into functional hepatocytes by directed evolution of native hepatic reprogramming factors (>link 8)
